Chlorine is the most commonly used chemical element for the elimination of micro-organisms and pathogens from swimming pool water, whether they are private pools or belong to water parks, hotels and resorts, campsites or other leisure and tourism facilities.
Regardless of the form of chlorine used (sodium hypochlorite, chlorine gas, calcium hypochlorite, etc.), when it comes into contact with water it forms hypochlorous acid (HClO). Hypochlorous acid dissociates producing a hypochlorite anion (ClO-) and a hydrogen cation (H+), according to the following reaction:
HClO <—> ClO–+ H+
The degree of dissociation and the equilibrium of this reaction depends on the pH value of the water.
Both forms of chlorine, hypochlorite and hypochlorous acid, have a high disinfecting power and constitute what is known as free chlorine or active chlorine.
The process of disinfection of swimming pool water consists of the oxidation of nitrogenous organic matter, in the form of micro-organisms such as algae, bacteria, germs, etc., or inert matter such as ammonia or urea, when they come into contact with chlorine. As a result of this oxidation, the combination of chlorine and organic matter generates what is known as chloramine.
Initially, monochloramines are formed, according to the following chemical reaction:
HClO + NH3 —> NH2Cl + H2O
If more chlorine is added to the water, already containing monochloramines, dichloramines are formed:
HClO + NH2Cl —> NHCl2 + H2O
Finally, with a reaction similar to the previous ones, trichloramines are formed:
HClO + NHCl2 —> NCl3 + H2O
Chloramines have a very low disinfectant power, and their presence causes irritation and bad smells. As we will see in the following graph, excess chloramine is also a reason for inefficiency, since it reduces the disinfecting power of chlorine.
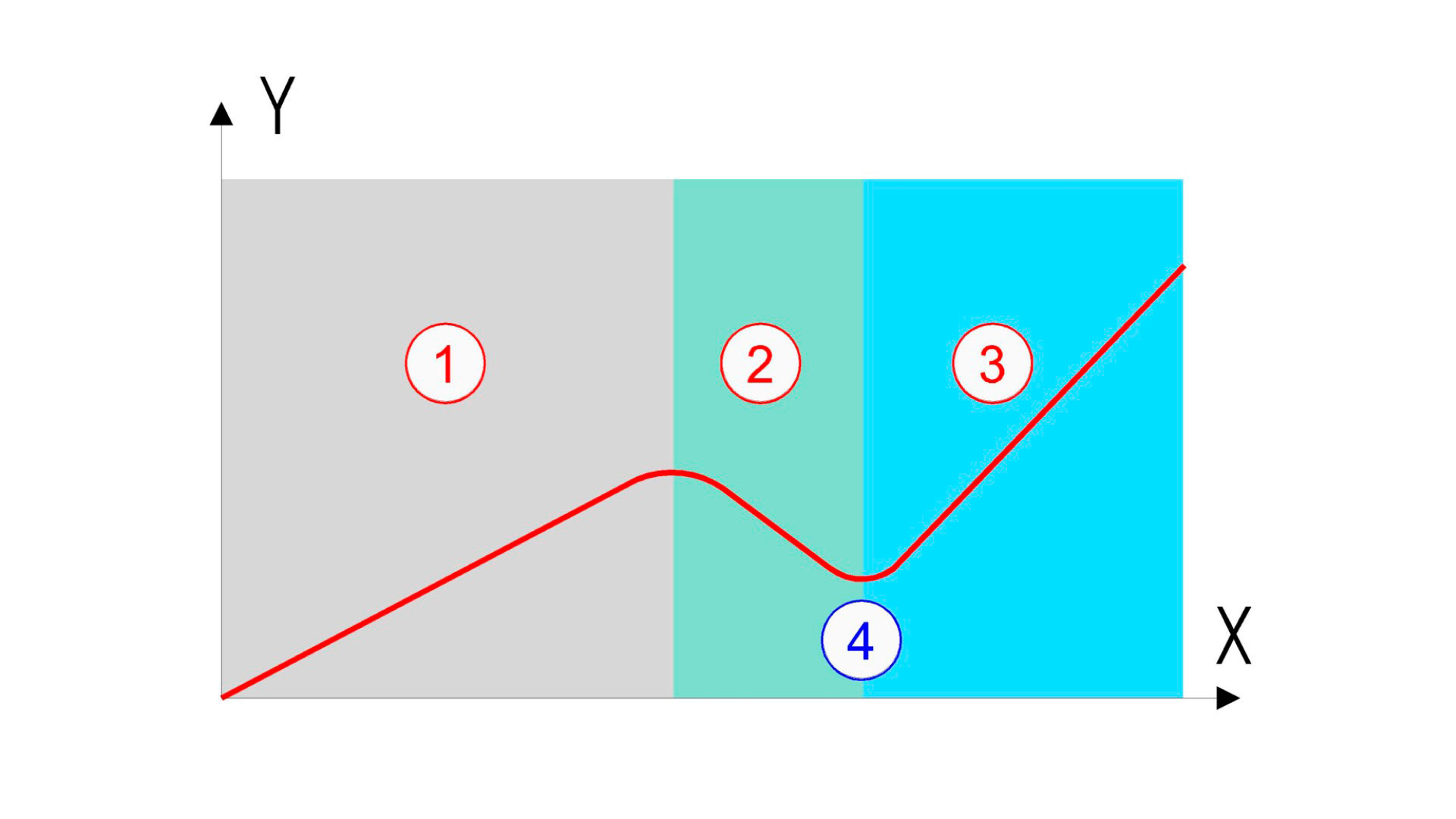
El eje X del gráfico corresponde a la concentración de cloro total dosificado, y el eje Y representa el cloro residual.
The X-axis of the graph corresponds to the dosed total chlorine concentration and the Y-axis represents the residual chlorine
When it combines with the organic matter in the water, active chlorine is transformed into chloramines (zone 1 in the graph) instead of carrying out its disinfectant function. Once a certain concentration of chlorine is reached, it begins to destroy these chloramines (zone 2 in the graph). As more chlorine is added, the chloramines are almost completely neutralised, so that free chlorine begins to remain in the water (zone 3 in the graph), when the so-called “break point” of chlorine is reached. The break point (marked with the number 4 in the graph) corresponds to the minimum dose of chlorine that must be reached in order to guarantee the existence of free chlorine in the water and, therefore, its effective disinfection in the pool.
Obviously, the lower the concentration of chloramines in the water, the lower the addition of chlorine required to reach the breakpoint. Consequently, and because the removal of chloramines is complex, some local regulations require the daily replacement of part of the pool water with drinking water in order to lower the concentration of chloramines and therefore ensure adequate disinfection.
However, for aquatic facilities such as water parks, hotel and resort pools or campsites, Amusement Logic proposes more sustainable solutions. Among them, it recommends operators to use the water from this regulatory emptying for cleaning the filters of the hydraulic system. Moreover, this water can still be dechlorinated afterwards and reused for irrigation of landscaped areas.
We have already dealt with this last issue in a series of previous articles, which we leave here in case you wish to read more about it:
»Water de-chlorination by aeration
» Water de-chlorination by forced aeration: a case study
By Luis Llor, Senior Hydraulic Engineer in the Architecture Department of Amusement Logic






