Ozone is an unstable gas, composed of 3 oxygen atoms, so its chemical formula is O3. It is highly oxidising and therefore useful for disinfecting, purifying and eliminating microorganisms such as viruses, bacteria and any other type of organic matter.
How ozone is generated
To generate ozone, air is passed through an electric field of high potential (image 1), so that the oxygen in the air, O2, is transformed into O3 (image 2). The normal concentration of ozone in ozonised air is 10 to 20 g/m³. If the gas used for ozone generation is oxygen instead of air, the concentration can be twice as high as that obtained from air.
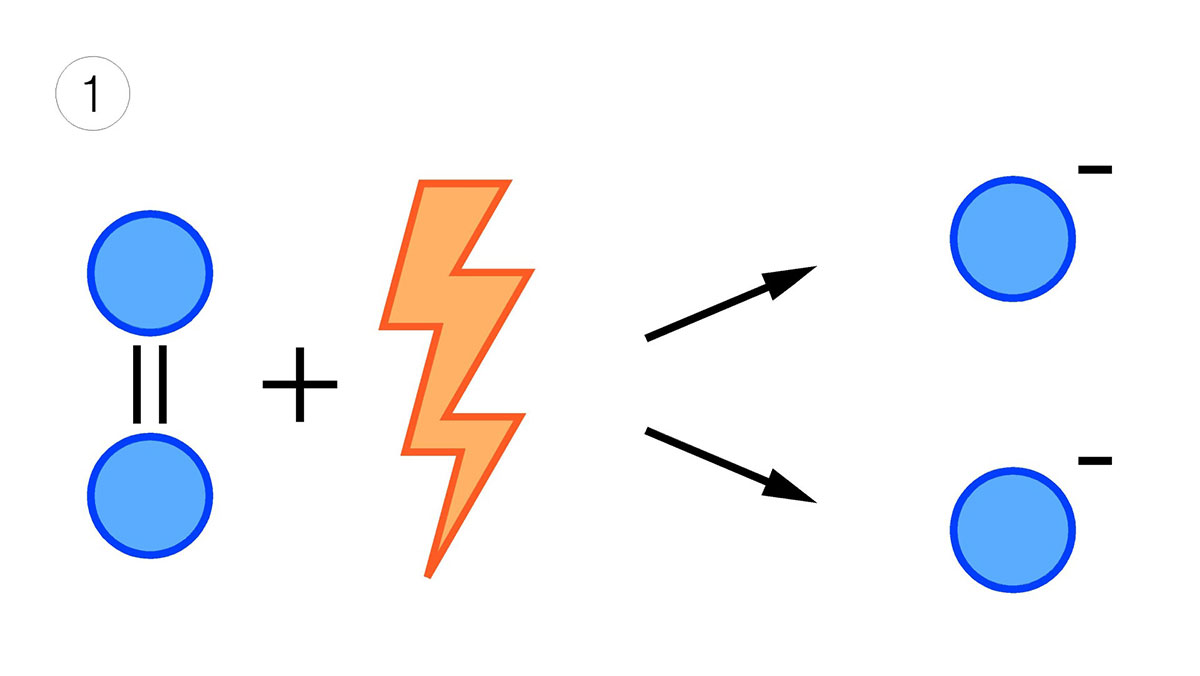
Image 1: Splitting of the oxygen molecule by an electric field.
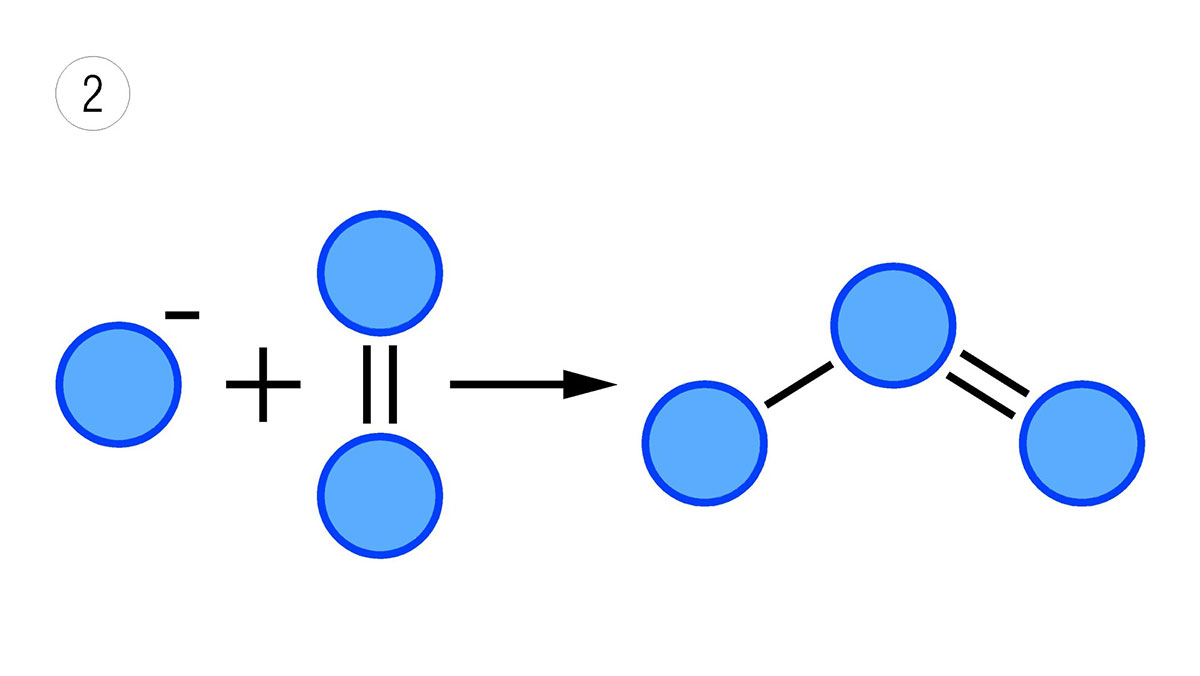
Image 2: The combination of ion and oxygen molecule gives rise to the ozone molecule.
The industrial production of ozone is carried out in ozonators. Due to the instability of ozone, in order to guarantee its disinfecting effect, it is injected into the water as soon as it is generated.
Disinfecting action of ozone in swimming pool water
When ozone is injected into the water, the gas exerts its oxidising power by two mechanisms:
–Direct oxidation of organic compounds is produced by molecular ozone.
-Or, oxidation occurs by means of hydroxyl free radicals (OH-), formed when ozone is injected into the water, according to the following formulas:
O3 + H2O → HO3++OH–
HO3+ + OH– → 2HO2
Hydroxyl free radicals have an oxidation potential of 2.80 V, whereas the potential of ozone is 2.07 V. In other words, the disinfecting power of hydroxyl is much higher than that of molecular ozone. In fact, it is one of the most powerful oxidants in water. Hypochlorous acid, which is the most common disinfectant used in swimming pools, has a redox (oxidation and reduction) potential of 1.49 V, which is considerably lower than the former.
The disadvantage of hydroxyl for disinfection purposes is that its lifetime in water is microseconds. However, this is sufficient time for it to exert its oxidising power and disinfect the water.
Advantages of swimming pool water disinfection using ozone
–Greater oxidising power than other products.
–It does not produce trihalomethanes, but removes their precursors.
–It does not alter the pH of the water.
–It improves the coagulation phenomenon.
–Reduces odours and tastes in the water.
-It facilitates the removal of iron and manganese.
Disadvantages of ozone in the disinfection of swimming pool water
-The initial investment to install an ozone disinfection system is higher than for a conventional system. The costs of operation and generation of ozone, due to the high consumption of electrical energy, are also higher than in traditional systems.
-It does not maintain a permanent residual concentration in the water. This makes it necessary to also use disinfectants such as sodium hypochlorite, in order to guarantee a minimum residual concentration of disinfectant.
-It can form some harmful products, such as bromates, aldehydes, or nitric acid, which cause corrosion of equipment.
For all these reasons, the installation of an ozone disinfection system in water parks and swimming pools should be carefully studied.
By Luis Llor, Senior Hydraulic Engineer in Amusement Logic’s Architecture Dept.






